Cell-free protein synthesis is the production of proteins “outside of the cell” using only DNA, cell-free reagents, and buffers. It has always held promise for its speed and simplicity. However, two common complaints of the technology have been its inability to make complicated proteins, and its inability to scale.
Most companies today produce and sell cell-free protein synthesis reagents produced from E. coli, which is recognized to be high-yield and easier to produce but limited in its ability to make more complex proteins. Cell-free reactions run at 1,000 L scale have been publicly shown by Sutro Biopharma. There are similar, but non-public, scales achieved by other players in the field from Vaxcyte/Lonza and Resilience.
However, a company you have likely never heard of claims to have demonstrated 100,000 L capacity. More impressively, they claim to have done this using a non-E. coli, yeast-based cell-free system.

The company is called Kangma Healthcode (康码(上海)生物科技有限公司). It was founded in 2015, and is based in Minhang, a Shanghai suburb. It has almost no Western presence, and is little known about the company outside of China. However, according to Tianyancha it was valued at $2.8B RMB in 2022, or $390M USD, and has over 200 employees.
Its CEO and founder is Min Guo, a scientist by training. Guo trained at the Scripps Institute from 2005-2016, first as a post-doc with Paul Schimmel before becoming an Associate Professor. He returned to China in 2016 to start Kangma. Guo’s academic resume is impeccable, with first-author publications in Nature and Science. However, prior to starting Kangma Guo had no publications or training in cell-free protein synthesis. From interviews given to Chinese press, Guo started building Kangma from the ground-up with a team of 10.
Kangma has reportedly solved two challenging technical problems: cell-free protein synthesis using yeast, and industrial (300,000 L) scale-up. Wrapped around this is work on commercialization and standardization. That it has done all of this in 8 years is impressive, given the known long time-scales for cell-free technology development.
A productive yeast cell-free system
The first problem, of basic science, is producing a high-yield yeast system. Yeast is particularly valuable as a eukaryotic microorganism because it can produce proteins that are difficult to make in prokaryotic systems. This is due to its use of various chaperones and its organelles, which help ensure proper protein folding and post-translational modification. Other eukaryotic systems exist, such as LenioBio’s ALICE™ Tobacco BY2 system and CellFree Sciences’ Wheat Germ system. However, yeast has the added benefit of being easier to scale using well-established microbial culturing and processing techniques.
A productive yeast system has been elusive in the Western world. Work has been done in S. cerevisiae (Jewett lab at Stanford) and P. pastoris (Polizzi lab at Imperial College London, Zamella lab at Fraunhofer, among others), with yields approaching ~ 0.1 mg/ml in batch mode.
Kangma self-reports ~0.2 mg/ml to 1 mg/ml of production. Those ranges are in-line with the fluorescent protein production yields by color shown on their website. Kangma’s intellectual property library consists of almost 100 patents and counting. From this library, significant work has gone into buffer engineering to enable central metabolism (building off of seminal work here), optimizing translational regulation (eg. optimizing Pab1-eIF4G with novel approaches), strain engineering (eg. removing proteases with novel approaches), and putative selection of the K. lactis yeast strain as a starting point. However, caveats are necessary as there is no peer-reviewed work on the system.
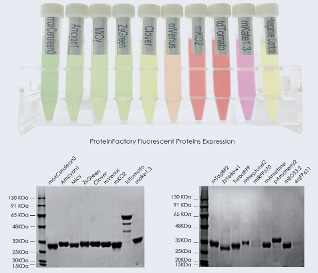
Scaling to 100,000 L
The second problem, of bioprocessing, is scale-up to 100,000 L. Kangma hit ~ 5,000 L (5 ton) scale in 2020, followed by ~100,000 L (100-ton) scale in 2024. It is unclear if these numbers are for producing the starting reagent or for the final protein synthesis reactor volume. Also unclear is how much of the facility is GMP certified, but videos from inside the facility suggest very advanced development and GMP-like process. The scales indicate significant bioprocessing capability and rapid process innovation versus the current Western industry standard of a 1,000 L scale cell-free reaction. Part of succeeding here requires solving additional technology development problems, as must current cell-free scale-up processes are optimized for E. coli, not yeast. Innovations like a novel nitrogen grinding lysis method are likely required to achieve this scale.
Commercialization
Wrapped around Kangma’s technical innovations are rapid commercialization and standardization of cell-free technologies. Their website, www.ourproteinfactory.com, shows multiple cell-free kits available for sale, optimized for different applications (eg. standard, fast, high-yield, membrane, SPR, non-standard amino acid incorporation). These kits are sold lyophilized and produced in standard form on a factory line. Both of these are industry-leading innovations.

120 mL of their FAST kit, for example, sells for 372 RMB ($52 USD). This equates to a price of $0.43/mL, which is almost ~1000x cheaper than the industry-leading NEB S30 E. coli cell-free kit which sells for $1,922 USD for 5 mL, or $384/mL. The price differential allows the technology to jump from one reserved for hard-to-express proteins to a technology that competes with cellular recombinant production.
Kangma also sells molecular biology purified enzymes and kits (eg. polymerases, nucleases, etc.), regents needed for protein purification (eg. affinity binding beads, gels), and even an instrument to auto-purify protein. Presumably much of this is enabled by their cell-free production capacity.
In other areas, Kangma has publicized its prior commercialization of an ACE2 inhibitor Kansetin and its upcoming commercialization of hemoglobin for artificial blood, proteins and enzymes for the cosmetics market, and next-generation therapeutic proteins such as GLP-1 variants. Specifically for GLP-1, Kangma recently expanded its production to a new site, Xishan Synthetic Biology Industrial Park in Wuxi.
Why should I care?
Kangma’s work shows us that cell-free protein synthesis can be industrialized and scaled, and that outsiders to the field can rapidly make progress by leveraging existing knowledge in new ways. At $1/mL, using cell-free protein synthesis to produce measurable and usable amounts protein was rarely feasible. Kangma's scale, translated into accessibility by its pricing, democratizes cell-free protein synthesis into the mainstream.
Kangma's technical advances, if validated, likely push the field forward 5-10 years from where it is now, and unlocks new opportunities. Industrially and commercially, next up is demonstrating that multiple commercially viable targets can be produced once cell-free is scaled, especially outside of the therapeutic modalities of ADCs (Sutro) and conjugate vaccines (Vaxcyte). Academically, Kangma opens the door to new and improved “frontier” cell-free systems based on Kangma’s technological leaps in yeast cell-free biochemistry.
Zachary Sun is the Founder of Sepia Biosciences and Tierra Biosciences. Opinions represented are of his own.



This is great coverage and an impressive feat. I can’t wait for more labs to transition to cell-free protein expression, makes no sense using bacterial hosts for all the work.